TIM-3 Therapy for Alzheimer’s offers a promising new avenue for treating Alzheimer’s disease, leveraging strategies traditionally used in cancer immunotherapy. Recent studies have illuminated the role of the TIM-3 protein, an immune checkpoint molecule that, when inhibited, allows microglia to effectively remove amyloid plaques from the brain. This breakthrough has shown significant potential for cognitive improvement in Alzheimer’s, as evidenced by improved memory performance in mouse trials. Researchers are optimistic that anti-TIM-3 therapies could emerge as a transformative approach in Alzheimer’s disease treatment, targeting the underlying mechanisms that inhibit plaque clearance. As we delve further into the TIM-3 gene study, the implications for future therapies could reshape how we combat this devastating condition.
In the realm of Alzheimer’s disease management, innovative strategies like TIM-3 Therapy are making waves by repurposing immune checkpoint therapies originally designed for cancer treatment. By experimenting with the TIM-3 molecule, scientists have uncovered a link between this gene and late-onset Alzheimer’s, paving the way for novel interventions. The concept revolves around restoring the function of microglia, the brain’s immune cells, enabling them to eliminate harmful amyloid plaques that contribute to cognitive decline. As researchers explore this cutting-edge approach, it raises hope for patients seeking more effective solutions, especially in light of previous challenges faced in Alzheimer’s disease treatment. With such advancements, we stand on the precipice of significant breakthroughs that could fundamentally change the landscape of Alzheimer’s interventions.
Understanding TIM-3’s Role in Alzheimer’s Disease
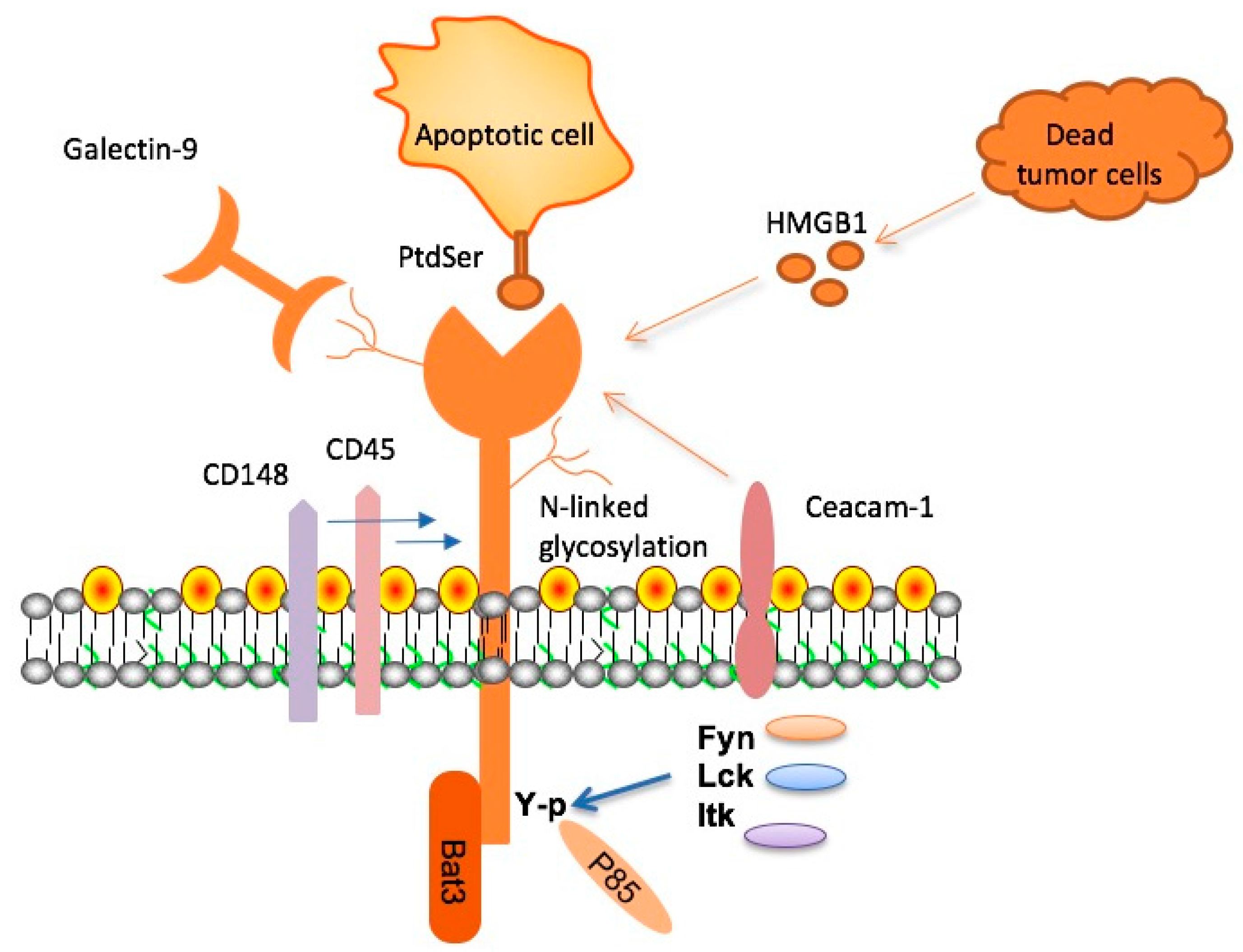
TIM-3, an immune checkpoint molecule, plays a crucial role in regulating the immune response within the brain. In Alzheimer’s disease, particularly in late-onset cases, its expression becomes significantly elevated on microglia, the brain’s resident immune cells. This increase restricts microglia from clearing amyloid plaques, which contribute to the cognitive decline observed in Alzheimer’s patients. Understanding the mechanisms behind TIM-3’s activity not only highlights its role as a genetic risk factor but also underscores its potential as a therapeutic target for enhancing the immune response to Alzheimer’s pathology.
Research shows that when TIM-3 is inhibited, microglia can effectively engage with amyloid plaques, facilitating their clearance. This change is essential because excessive accumulation of these plaques leads to neuronal damage and memory loss. By targeting TIM-3, scientists hope to create therapeutic strategies that reactivate the innate functions of microglia, allowing them to properly manage amyloid burden in the brain and potentially restore cognitive function, providing hope for Alzheimer’s disease treatment.
Immune Checkpoint Therapy and Cognitive Improvement
The concept of immune checkpoint therapy, traditionally used in cancer treatment, is now being explored in the context of Alzheimer’s disease. This approach focuses on modulating the immune response to improve cognitive outcomes. By blocking checkpoint molecules like TIM-3, researchers have noted significant improvements in memory and overall cognitive abilities in preclinical models of Alzheimer’s. These findings suggest a paradigm shift in how we approach the treatment of neurodegenerative diseases, potentially introducing immune checkpoint blockade as a viable therapeutic strategy.
Studies have demonstrated that in mice genetically modified to lack TIM-3, microglia showed enhanced ability to clear amyloid plaques, leading to observable cognitive enhancements. The cognitive assessment in these mice highlighted that memory retrieval improved significantly when plaques were reduced, indicating that immune modulation can effectively counteract some of the pathological features of Alzheimer’s disease. As a result, the application of immune checkpoint therapy could represent a novel frontier in promoting cognitive health in Alzheimer’s patients.
Microglia: The Immune Guardians of the Brain
Microglia are often referred to as the immune guardians of the brain, playing a pivotal role in maintaining homeostasis and responding to injury. These cells are responsible for clearing cellular debris and plaques through phagocytosis, processes that are critical for brain health. However, in Alzheimer’s disease, the functionality of microglia is compromised due to the overexpression of inhibitory molecules like TIM-3, which prevents them from effectively engaging amyloid plaques and performing their protective roles.
Understanding the dual nature of microglia—both as defenders against cognitive decline and as suppressors of immune activity—offers insight into potential treatment avenues. By manipulating the expression of molecules like TIM-3, researchers could help restore microglial function, enhancing their ability to manage plaque burden and protect against neurodegeneration. This research emphasizes the importance of microglia and their potential targeting in future Alzheimer’s disease interventions.
The Promise of TIM-3 Therapy for Alzheimer’s Patients
TIM-3 therapy holds significant promise as a novel treatment avenue for Alzheimer’s disease. By utilizing anti-TIM-3 antibodies or small molecules that block TIM-3’s inhibitory function, researchers aim to unleash the potential of microglia to combat amyloid plaques effectively. This approach directly addresses the underlying mechanism contributing to cognitive decline in Alzheimer’s patients, making it a potentially groundbreaking therapy in the field of neurodegenerative disease management.
Preclinical studies have laid the groundwork for TIM-3 targeted therapies, demonstrating enhanced memory function and plaque clearance in mouse models. As research progresses, the goal is to translate these findings into human clinical trials, offering hope for patients with Alzheimer’s. By focusing on existing immune checkpoint therapies, there is a pathway to fast-track new interventions that could provide cognitive improvements and restore some quality of life to those afflicted by this challenging disease.
Genetic Insights from TIM-3 Gene Studies
The TIM-3 gene (HAVCR2) provides vital genetic insights into the susceptibility to late-onset Alzheimer’s disease. Variations within this gene have been associated with increased risk, emphasizing the importance of genetic profiling in understanding the complexities of Alzheimer’s. Studies have shown that individuals with certain TIM-3 polymorphisms exhibit enhanced levels of the molecule on microglia, linking it directly to the disease pathology.
Exploring the genetic underpinnings of TIM-3 not only elucidates its role in Alzheimer’s but also opens doors for personalized therapy approaches. By understanding which genetic profiles are most affected by TIM-3 expression, clinicians may better tailor interventions, potentially leading to improved outcomes for patients based on their specific genetic risk factors. This strategy aligns with the increasing focus on precision medicine in the treatment of neurological disorders.
The Mechanism of Amyloid Plaque Accumulation
Amyloid plaques form due to the improper clearance of amyloid-beta peptides, a process that is significantly affected by microglial dysfunction. In Alzheimer’s disease, the accumulation of these plaques correlates with the cognitive decline seen in patients. The presence of TIM-3 on microglia inhibits their normal phagocytic activity, allowing these harmful plaques to persist in the brain.
This understanding of the mechanisms behind amyloid plaque accumulation has led researchers to explore ways to enhance microglial function. By targeting TIM-3, scientists aim to restore the ability of microglia to clear amyloid-beta, potentially reversing some of the cognitive impairments associated with Alzheimer’s. The effort to elucidate these mechanisms is crucial for developing therapies aimed at improving cognitive outcomes in Alzheimer’s patients.
Navigating the Challenges of Alzheimer’s Drug Trials
Alzheimer’s disease drug trials have faced numerous challenges, with many candidates showing limited efficacy in improving cognitive outcomes. Recent successes highlight a new focus on specific molecular targets, such as TIM-3, rather than broad approaches that may not address the underlying mechanisms of the disease. By concentrating on targeted therapies that can enhance microglial activity, researchers hope to overcome the setbacks of previous trials and deliver meaningful benefits to patients.
The key to navigating future Alzheimer’s drug trials lies in understanding the complex biology of the disease and the role of immune checkpoints like TIM-3. Developing therapies that successfully modulate this pathway may provide breakthrough treatments that not only halt disease progression but also promote cognitive recovery. As the medical community learns from past trials, the integration of novel targets will be critical in reshaping the landscape of Alzheimer’s disease treatment.
The Future of Alzheimer’s Disease Treatment
The future of Alzheimer’s treatment is promising, with emerging research highlighting the value of innovative approaches such as TIM-3 therapy. As trials begin to explore the therapeutic potential of immune checkpoint modulation, there is hope that these strategies will yield significant advancements in patient care. By harnessing the power of the immune system, researchers aim to create treatments that not only target the symptoms of Alzheimer’s but also address the disease’s root causes.
Future studies will focus on further elucidating the roles of TIM-3 and other immune checkpoint molecules in Alzheimer’s. Continued exploration of these pathways will aid in developing cutting-edge therapies that could transform the standard of care. As our understanding of Alzheimer’s disease deepens, the integration of genetic insights and immunological strategies will be fundamental in creating effective treatment modalities that enhance cognitive function and quality of life for patients.
Frequently Asked Questions
What is TIM-3 therapy for Alzheimer’s disease treatment?
TIM-3 therapy for Alzheimer’s disease treatment utilizes an immune checkpoint molecule called TIM-3 to enhance microglial activity in clearing amyloid plaques from the brain, which may improve cognitive functions in Alzheimer’s patients.
How does TIM-3 impact microglia and Alzheimer’s progression?
In Alzheimer’s disease, TIM-3 restricts microglia from effectively removing harmful amyloid plaques. By inhibiting TIM-3, therapy aims to restore microglia’s ability to clear these plaques, potentially slowing down Alzheimer’s progression.
What are the potential benefits of TIM-3 therapy for cognitive improvement in Alzheimer’s?
Potential benefits of TIM-3 therapy for cognitive improvement in Alzheimer’s include enhanced clearance of amyloid plaques, reduction in neuroinflammation, and possible restoration of memory function, as seen in mouse models.
Is there ongoing research on TIM-3 gene study related to Alzheimer’s disease?
Yes, ongoing research on the TIM-3 gene study is investigating its role as a genetic risk factor for late-onset Alzheimer’s and how manipulating TIM-3 expression could lead to novel treatment strategies.
Can TIM-3 therapy be used in combination with existing Alzheimer’s treatments?
TIM-3 therapy could potentially be used alongside existing Alzheimer’s treatments, particularly if it demonstrates efficacy in enhancing plaque clearance without substantial vascular damage.
What is the significance of the TIM-3 polymorphism in Alzheimer’s patients?
The TIM-3 polymorphism is associated with higher expression of TIM-3 in microglia in Alzheimer’s patients, which may prevent proper plaque clearance and contribute to disease severity.
How does immune checkpoint therapy relate to Alzheimer’s disease and TIM-3?
Immune checkpoint therapy, like TIM-3 therapy, aims to modulate the immune response to enhance the clearance of amyloid plaques in Alzheimer’s, similar to strategies used in cancer treatment.
What animal models are used to test TIM-3 therapy for Alzheimer’s?
Research in TIM-3 therapy for Alzheimer’s uses genetically modified mouse models that lack the TIM-3 gene, allowing scientists to observe changes in plaque clearance and cognitive function.
What are the next steps in TIM-3 research for Alzheimer’s treatment?
Next steps in TIM-3 research for Alzheimer’s treatment include testing humanized anti-TIM-3 antibodies in mouse models to evaluate their effectiveness in halting plaque development and improving cognitive outcomes.
How does TIM-3 therapy compare to traditional Alzheimer’s drug trials?
Unlike traditional Alzheimer’s drug trials that focus on amyloid-beta, TIM-3 therapy offers a novel approach by targeting the immune response, potentially circumventing some limitations observed with current treatment methods.
| Key Points |
|---|
| Vijay Kuchroo and his team conducted a study on TIM-3 for Alzheimer’s treatment. |
| 90% – 95% of Alzheimer’s cases are late-onset, linked to the TIM-3 gene. |
| TIM-3 acts as a checkpoint molecule that inhibits microglia from clearing amyloid plaques in the brain. |
| Deletion of TIM-3 in mice improved plaque clearance and cognitive function. |
| Potential therapies using anti-TIM-3 antibodies could help treat Alzheimer’s in humans. |
| Future work aims to test human anti-TIM-3 in mouse models of Alzheimer’s disease. |
Summary
TIM-3 Therapy for Alzheimer’s is a promising area of research that explores how inhibition of the TIM-3 checkpoint molecule can enhance the brain’s ability to clear amyloid plaques. With most Alzheimer’s cases being late-onset, research suggests that blocking TIM-3’s inhibitory effects on microglia could lead to better cognition and memory restoration. Current studies in mouse models show that removing TIM-3 expression allows microglia to effectively eliminate plaques, which may pave the way for innovative therapies in humans. As researchers continue to explore anti-TIM-3 therapies, there is hope for more effective treatments against Alzheimer’s, marking a significant step forward in combating this challenging disease.